Binary mixture
➙ Mixture of two or more misible liquids
➙ Binary mixture is kind of homogenous mixture in which two or more liquids are completely miscible with each others.
➙ Boiling point of mixture is depend on composition of solution all the mixture fall in 3 classes.
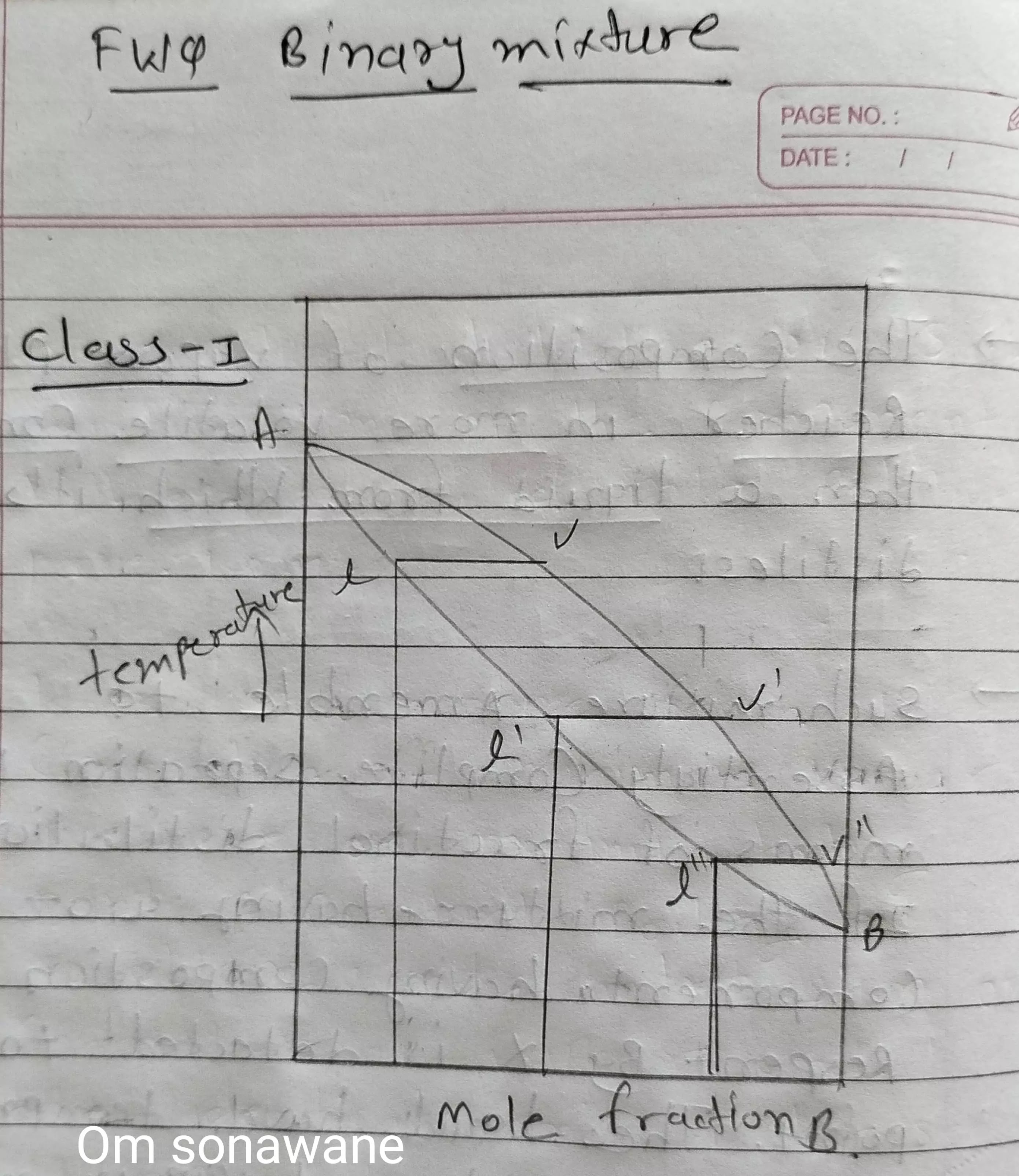
Class 1 Binary mixture :
➙ Boiling point of binary mixture is between boiling point of two components of mixture.
➙ The vapour pressure of a mixture is less than most volatile and more than list volatile components.
➙ Composition of vapour always Richard in more volatile compound then a liquid from which it's distilles.
➙ Composition of vapour always Richard in more volatile compound then a liquid from which it's distilles.
➙ Such mixture amenable to essentially complete separation by means of fractional distillation.
➙ If the mixture having A & B components having composition represent by X is detected to boiling point.
➙ The liquid have temperature composition 'L' and vapour produce will have composition corresponding all the vapour curves.
➙ If the vapour at we condense a liquid corresponding 'X' much Richard in the octane.
➙ Distillation of the mixture (X') resulting in the vapour with composition (X") through successful condensation and evaporation a distillation of essentially your (B) having of obtain.
➙ (A) will remain relatively pure residue in the reactor.
Class 2 Binary mixture:
➙ Class 2 binary mixture includes mixer that at a certain Mall ratio have vapour pressure less than either of the compounds at this mole ratio the mixture will have boiling point greater than other of the composition.
➙ Confectional distillation of such mixture one up the components will be fascinate into relatively your form.
➙ When liquid mixer beach is composition minimum vapour pressure or maximum boiling point ; constant boiling mixture is obtained and of composition of vapour and liquid are identical and further separation is impossible.
➙ The diagram show composition of liquid and vapour phase class 2 binary mixture.
➙ Is corresponding to X' is the still the vapour from is which a component A can we recover in parts in relatively pure form.
➙ As a remove from the mixture the liquid phase until the equal composition show X.
➙ At this point composition of liquid at vapour phase are same and constant volume mixture cannot be frictionated.
➙ Similarly, if a liquid composition X" is distillated distillation B is obtained and liquid phase rich composition = X , and a constant volume mixture will result.
➙ Example, hydrochloric, hydrobromic, nitric and formic acid is aqua solution are binary mixture class 2.
Class 3 Binary mixture :
➙ Binary mixture includes those mixer that at certain Mall ratio have vapour pressure greater than other of the components.
➙ Boiling point of mixture at this Mall ratio are lower than either of the component upon distillation and distillation obtaine d that contains.
➙ Both components in constant ratio and residue remind in class consist of one or other components in pure form.
➙ A diagram show in components of liquid and vapour phase of class 3 binary mixture is given.
➙ A corresponding to X' to produce a vapour with composition V'
➙ Fractional of this vapour will produce distillated with composition X and liquid face will grow richer component A
➙ Distillation of this mixture corresponding X" will produce a paper with composition V' fractionation yield a distillation composition X and unlike with will decompression Richard with compound B.
➙ Either component pure A and pure B will remain in liquid phase.
➙ Ethanol and water form mixture of class 3



.jpg)

0 Comments