Conductivity of an Electrolyte solution is measured of its ability to conduct electricity.
The SI unit of electrical conductivity is siemens per metre (S/m).
Conductivity of river water is high as compared to sea water ( everything depend on ions )
There are Three Types of conductivity :
- Table type conductivity
- Flow type conductivity
- Pipette type conductivity
Important Questions from Ma'am :
- What is the relation between conductivity and total dissolve solid.
- Derive the equation to find specific conducterts it's cell constant.
- Explain principle of conductivity explain the components and working with neat sketch.
- List different types of conductivity cells depend on their use.
- Highlight the significance of conductivity in water and wastewater.
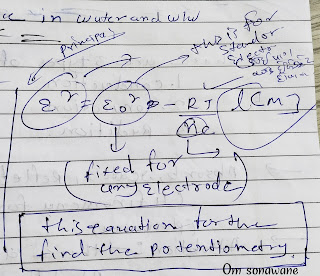
Potentiometry
Is the technique that is used analytical chemical usually to find the concentration of solute in solution.
- Metal metal ion Electrode (Ag/ag+)
- Metal metal salt Electrode (Ag/Agcl+)
- Oxidation Reduction Electrode ( Fe+2/Fe+3)
- Metal metal amalgon (Hg/ Hg2cl2)
- Gas electrode ( PT/H2 )
There are Two Types of Potentiometry instruments are used :
- Ion selective method ( alkali, fluorine, fluoride, inert measurement )
- pH meter Method
Membrane
There are 5 types of Membrane :
- Ion exchange membrane
- Polymeter membrane
- Cellulose membrane
- Glass membrane
Important Questions from Ma'am:
- Define potentiometry
- Explain the principle of an selective electrode
- List different types of electrode used in electrical method of measurement briefly explain with figure.
- List different membrane used in measuring electrode.
- What is reference electrode explain.
- Explain standard celonometer with figure.


.jpg)

0 Comments