Membrane Filtration
Membrane filtration is a physical separation method that uses a membrane to separate molecules of different sizes and characteristics. The membrane acts as a physical barrier with highly specialized characteristics that only certain selected components in the feed stream can pass through.The driving force behind the separation process is the difference in pressure between the two sides of the membrane
Membrane filtration is a clean technology that can bring down overall production costs and boost product quality at the same time. It is a rapidly expanding field in water treatment, with many different types of filters available in a wide range of pore sizes. Membrane filtration is used in a wide range of applications, ranging from dairy processing to wastewater treatment.
There are different types of filter membranes, including reverse osmosis (RO) membranes, ultrafiltration (UF) membranes, and nanofiltration (NF) membranes. Each type approaches the membrane filtration process a little bit differently. For example, reverse osmosis applies pressure to a semipermeable membrane that allows the water molecules to pass through while flushing the dissolved inorganic compounds to the drain. Ultrafiltration, on the other hand, doesn't separate the water like a reverse osmosis system, but instead separates particles from the water.
Membrane filtration is also used as a technique for testing water samples. In this procedure, water is drawn through a special porous membrane designed to trap microorganisms larger than 0.45μm. The filter is then applied to the surface of Endo agar plates and incubated for 24 hours.
Membrane Process Terminology :
- Tubular membrane: Membranes in a tube form with the membrane casted on the inside diameter that are used for micro- and ultrafiltration.
- Permeate: The filtrate from a membrane filter. It is called permeate due to the way that the feed water permeates through the membrane.
- Wetting: A term that describes how easily membranes can be wetted, as well as its ability to resist fouling to some degree.
- Fouling: The accumulation of unwanted material on the surface of a membrane, which can reduce its effectiveness.
- Pressure-driven membrane filtration: A method of separating particles in liquid solutions or gas mixtures. This technique is used in a wide range of applications ranging from dairy processing to wastewater treatment.
- Cross-flow membrane filtration: A type of pressure-driven membrane filtration process where the feed stream flows parallel to the membrane surface, which helps to reduce fouling and increase the efficiency of the process.
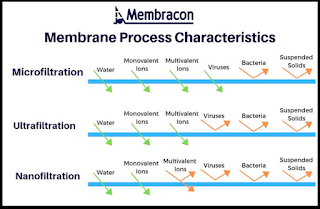
- Microfiltration: A type of filtration membrane that is commonly used as a pretreatment or clarification step prior to other membrane processes for the removal of fats and large macromolecules.
- Ultrafiltration: A type of filtration membrane that allows for the removal of all bacteria, as well as some viruses, and is commonly used throughout the dairy and food industry for the concentration of proteins and juices.
- Nanofiltration and reverse osmosis: A type of thin film composite membrane that is commonly used in desalination applications due to their ability to selectively remove salts and other dissolved solids from water.
Here are some of the most common membrane process terminology:
- Membrane: A thin film that allows some substances to pass through it while blocking others. Membranes are typically made of polymers, ceramics, or metals.
- Membrane separation: The process of separating different components of a mixture using a membrane.
- Membrane process: A specific type of membrane separation process. There are many different membrane processes, each with its own unique properties and applications.
- Membrane filtration: A membrane process that separates particles from a liquid or gas by size.
- Membrane distillation: A membrane process that separates a solvent from a solution by vapor pressure.
- Membrane electrodialysis: A membrane process that separates ions from a solution by applying an electric field.
- Membrane contactors: A membrane process that combines membrane filtration and membrane distillation.
Here are some additional terms that you may encounter when learning about membrane processes:
- Membrane selectivity: The ability of a membrane to allow some substances to pass through it while blocking others.
- Membrane permeability: The rate at which a substance can pass through a membrane.
- Membrane fouling: The accumulation of impurities on a membrane, which can reduce its selectivity and permeability.
- Membrane cleaning: The process of removing impurities from a membrane.
Membrane processes are a versatile and efficient way to separate different components of a mixture. They are used in a wide range of industries, including water treatment, food processing, and the chemical industry.
Here are some examples of how membrane processes are used in food processing:
- Microfiltration: To remove bacteria and other microorganisms from milk.
- Ultrafiltration: To remove proteins and other large molecules from milk.
- Nanofiltration: To remove salts from water.
- Reverse osmosis: To remove salts from seawater.
- Membrane distillation: To concentrate fruit juices.
Membrane processes offer a number of advantages over traditional separation methods, including:
- High selectivity: Membranes can be designed to allow only certain substances to pass through them, which can improve the purity of the final product.
- Low energy consumption: Membrane processes typically require less energy than traditional separation methods, such as distillation.
- Compact equipment: Membrane processes can be carried out in relatively small, compact equipment, which can save space.
Membrane processes are a growing field of technology with a wide range of potential applications. As the technology continues to develop, we can expect to see even more innovative and efficient membrane processes being developed in the future.
Membrane Process Classification and operation :
1. Microfiltration :
Classification:
Operation:
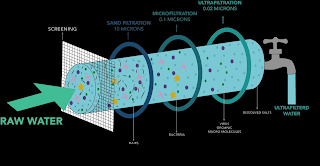
Microfiltration (MF) is a membrane process that separates particles from a liquid or gas by size. MF membranes have pore sizes that range from 0.1 to 10 micrometers (μm), which is large enough to allow water and small molecules to pass through, but small enough to block bacteria, viruses, and other microorganisms.
MF is a pressure-driven membrane process, which means that the feed solution is pumped across the membrane under pressure. The pressure gradient across the membrane drives the water and small molecules through the pores, while the larger particles are blocked.
MF is a versatile membrane process that has a wide range of applications. It is used in a variety of industries, including:
- Water treatment: MF is used to remove bacteria, viruses, and other microorganisms from drinking water, wastewater, and industrial water.
- Food processing: MF is used to remove bacteria and other microorganisms from milk, juices, and other food products.
- Chemical processing: MF is used to remove particles from chemicals and other industrial fluids.
MF is a relatively simple and efficient membrane process. It is relatively easy to operate and maintain, and it does not require the use of chemicals or heat. MF is also a relatively low-cost membrane process.
Here are some of the advantages of microfiltration:
- High selectivity: MF membranes can be designed to allow only certain particles to pass through, which can improve the purity of the final product.
- Low energy consumption: MF processes typically require less energy than traditional separation methods, such as distillation.
- Compact equipment: MF processes can be carried out in relatively small, compact equipment, which can save space.
Here are some of the disadvantages of microfiltration:
- Membrane fouling: MF membranes can become fouled by the accumulation of particles on the membrane surface. This can reduce the selectivity and permeability of the membrane, and it can also increase the pressure drop across the membrane.
- Membrane cleaning: MF membranes need to be cleaned regularly to remove foulants. This can be a time-consuming and costly process.
Overall, microfiltration is a versatile and efficient membrane process that has a wide range of applications. It is a good choice for applications where the removal of bacteria, viruses, and other microorganisms is important.
2. Ultrafiltration :
Classification:
Operation:
Ultrafiltration (UF) is a membrane process that separates molecules from a liquid or gas by size. UF membranes have pore sizes that range from 0.001 to 0.1 micrometers (μm), which is small enough to block proteins, colloids, and other large molecules, but large enough to allow water and small molecules to pass through.
UF is a pressure-driven membrane process, which means that the feed solution is pumped across the membrane under pressure. The pressure gradient across the membrane drives the water and small molecules through the pores, while the larger molecules are blocked.
UF is a versatile membrane process that has a wide range of applications. It is used in a variety of industries, including:
- Water treatment: UF is used to remove proteins, colloids, and other large molecules from drinking water, wastewater, and industrial water.
- Food processing: UF is used to remove proteins, colloids, and other large molecules from milk, juices, and other food products.
- Chemical processing: UF is used to remove proteins, colloids, and other large molecules from chemicals and other industrial fluids.
UF is a relatively simple and efficient membrane process. It is relatively easy to operate and maintain, and it does not require the use of chemicals or heat. UF is also a relatively low-cost membrane process.
Here are some of the advantages of ultrafiltration:
- High selectivity: UF membranes can be designed to allow only certain molecules to pass through, which can improve the purity of the final product.
- Low energy consumption: UF processes typically require less energy than traditional separation methods, such as distillation.
- Compact equipment: UF processes can be carried out in relatively small, compact equipment, which can save space.
Here are some of the disadvantages of ultrafiltration:
- Membrane fouling: UF membranes can become fouled by the accumulation of molecules on the membrane surface. This can reduce the selectivity and permeability of the membrane, and it can also increase the pressure drop across the membrane.
- Membrane cleaning: UF membranes need to be cleaned regularly to remove foulants. This can be a time-consuming and costly process.
Overall, ultrafiltration is a versatile and efficient membrane process that has a wide range of applications. It is a good choice for applications where the removal of proteins, colloids, and other large molecules is important.
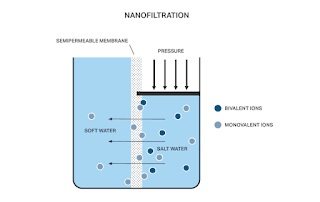
Nano - Filtration :
Classification:
Operation:
Nanofiltration (NF) is a membrane process that separates ions and other small molecules from a liquid or gas by size. NF membranes have pore sizes that range from 0.0001 to 0.001 micrometers (μm), which is small enough to block ions, salts, and other small molecules, but large enough to allow water and small molecules to pass through.
NF is a pressure-driven membrane process, which means that the feed solution is pumped across the membrane under pressure. The pressure gradient across the membrane drives the water and small molecules through the pores, while the larger molecules are blocked.
NF is a versatile membrane process that has a wide range of applications. It is used in a variety of industries, including:
- Water treatment: NF is used to remove ions, salts, and other small molecules from drinking water, wastewater, and industrial water.
- Food processing: NF is used to remove ions, salts, and other small molecules from milk, juices, and other food products.
- Chemical processing: NF is used to remove ions, salts, and other small molecules from chemicals and other industrial fluids.
NF is a relatively simple and efficient membrane process. It is relatively easy to operate and maintain, and it does not require the use of chemicals or heat. NF is also a relatively low-cost membrane process.
Here are some of the advantages of nanofiltration:
- High selectivity: NF membranes can be designed to allow only certain ions and molecules to pass through, which can improve the purity of the final product.
- Low energy consumption: NF processes typically require less energy than traditional separation methods, such as distillation.
- Compact equipment: NF processes can be carried out in relatively small, compact equipment, which can save space.
Here are some of the disadvantages of nanofiltration:
- Membrane fouling: NF membranes can become fouled by the accumulation of ions and molecules on the membrane surface. This can reduce the selectivity and permeability of the membrane, and it can also increase the pressure drop across the membrane.
- Membrane cleaning: NF membranes need to be cleaned regularly to remove foulants. This can be a time-consuming and costly process.
Overall, nanofiltration is a versatile and efficient membrane process that has a wide range of applications. It is a good choice for applications where the removal of ions, salts, and other small molecules is important.
1. Reverse Osmosis (RO):
Classification:
Operation:
Reverse osmosis (RO) is a membrane process that removes dissolved solids from a liquid by applying pressure to the liquid on one side of a semipermeable membrane. The pressure forces the water molecules through the membrane, while the dissolved solids are rejected.
RO is a versatile membrane process that has a wide range of applications. It is used in a variety of industries, including:
- Water treatment: RO is used to remove dissolved salts, minerals, and other contaminants from drinking water, wastewater, and industrial water.
- Food processing: RO is used to remove dissolved salts, minerals, and other contaminants from milk, juices, and other food products.
- Chemical processing: RO is used to remove dissolved salts, minerals, and other contaminants from chemicals and other industrial fluids.
RO is a relatively simple and efficient membrane process. It is relatively easy to operate and maintain, and it does not require the use of chemicals or heat. RO is also a relatively low-cost membrane process.
Here are some of the advantages of reverse osmosis:
- High selectivity: RO membranes can be designed to allow only certain dissolved solids to pass through, which can improve the purity of the final product.
- Low energy consumption: RO processes typically require less energy than traditional separation methods, such as distillation.
- Compact equipment: RO processes can be carried out in relatively small, compact equipment, which can save space.
Here are some of the disadvantages of reverse osmosis:
- Membrane fouling: RO membranes can become fouled by the accumulation of dissolved solids on the membrane surface. This can reduce the selectivity and permeability of the membrane, and it can also increase the pressure drop across the membrane.
- Membrane cleaning: RO membranes need to be cleaned regularly to remove foulants. This can be a time-consuming and costly process.
Overall, reverse osmosis is a versatile and efficient membrane process that has a wide range of applications. It is a good choice for applications where the removal of dissolved salts, minerals, and other contaminants is important.
Here are some additional details about reverse osmosis:
- The pressure required for reverse osmosis is typically in the range of 20 to 100 bar. The higher the pressure, the more water molecules will be forced through the membrane, and the more concentrated the brine (the rejected water) will be.
- The rate of water flow through a reverse osmosis membrane is called the permeate flux. The permeate flux is affected by the pressure, the temperature, and the salt concentration of the feed water.
- Reverse osmosis membranes are typically made of cellulose acetate, polyamide, or thin-film composite materials. Cellulose acetate membranes are the most common type of reverse osmosis membrane, but they are not as resistant to fouling as polyamide or thin-film composite membranes.
2. Electrodialysis:
Classification:
Operation:
Electrodialysis (ED) is a membrane process that uses an electric field to separate ions from a solution. The process is based on the principle that charged ions are attracted to oppositely charged electrodes. The feed solution is pumped through a series of cells, each of which contains a cation-exchange membrane and an anion-exchange membrane. The cation-exchange membrane allows cations to pass through, while the anion-exchange membrane allows anions to pass through. The electrodes are placed at the ends of the cells, and an electric field is applied across the cells. The cations are attracted to the negative electrode, and the anions are attracted to the positive electrode. This causes the ions to move through the membranes, and the separated ions are collected at the electrodes.
ED is a versatile membrane process that has a wide range of applications. It is used in a variety of industries, including:
- Water treatment: ED is used to remove ions from drinking water, wastewater, and industrial water.
- Food processing: ED is used to remove ions from milk, juices, and other food products.
- Chemical processing: ED is used to remove ions from chemicals and other industrial fluids.
ED is a relatively simple and efficient membrane process. It is relatively easy to operate and maintain, and it does not require the use of chemicals or heat. ED is also a relatively low-cost membrane process.
Here are some of the advantages of electrodialysis:
- High selectivity: ED membranes can be designed to allow only certain ions to pass through, which can improve the purity of the final product.
- Low energy consumption: ED processes typically require less energy than traditional separation methods, such as distillation.
- Compact equipment: ED processes can be carried out in relatively small, compact equipment, which can save space.
Here are some of the disadvantages of electrodialysis:
- Membrane fouling: ED membranes can become fouled by the accumulation of ions on the membrane surface. This can reduce the selectivity and permeability of the membrane, and it can also increase the energy consumption of the process.
- Membrane cleaning: ED membranes need to be cleaned regularly to remove foulants. This can be a time-consuming and costly process.
Overall, electrodialysis is a versatile and efficient membrane process that has a wide range of applications. It is a good choice for applications where the removal of ions is important.
Here are some additional details about electrodialysis:
- The electric field required for electrodialysis is typically in the range of 1 to 10 volts per centimeter. The higher the electric field, the more ions will be separated, and the more concentrated the brine (the rejected water) will be.
- The rate of ion transport through an electrodialysis membrane is called the current efficiency. The current efficiency is affected by the electric field, the temperature, and the ion concentration of the feed solution.
- Electrodialysis membranes are typically made of ion-exchange resins. Ion-exchange resins are polymers that have been chemically modified to have charged groups. The charged groups attract ions of the opposite charge, and this allows the ions to pass through the membrane.
Membrane Configurations:
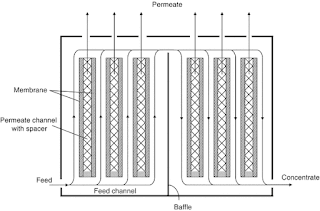
Plate-and-frame modules are a type of membrane module that is used in a variety of membrane processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis. Plate-and-frame modules are made up of a series of flat membranes that are sandwiched between plates. The plates are typically made of stainless steel or plastic, and they have spacers that create channels for the feed and permeate streams.
The feed stream flows through the channels between the plates, and the permeate stream flows through the membranes. The membranes allow certain substances to pass through, while blocking others. The permeate stream collects at the bottom of the module, and it is then pumped away.
Plate-and-frame modules are a versatile type of membrane module that can be used for a variety of applications. They are relatively easy to operate and maintain, and they are available in a wide range of sizes. However, plate-and-frame modules are not as compact as some other types of membrane modules, and they can be more expensive.
Here are some of the advantages of plate-and-frame modules:
- Versatility: Plate-and-frame modules can be used for a variety of membrane processes.
- Ease of operation: Plate-and-frame modules are relatively easy to operate and maintain.
- Wide range of sizes: Plate-and-frame modules are available in a wide range of sizes.
Here are some of the disadvantages of plate-and-frame modules:
- Size: Plate-and-frame modules are not as compact as some other types of membrane modules.
- Cost: Plate-and-frame modules can be more expensive than some other types of membrane modules.
Overall, plate-and-frame modules are a versatile and efficient type of membrane module that can be used for a variety of applications. They are a good choice for applications where the size and cost of the module are not a major concern.
Here are some details about spiral-wound modules:
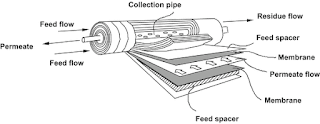
- Spiral-wound modules are a type of membrane module that is used in a variety of membrane processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
- The spiral-wound module consists of a stack of flat sheets of membrane material that are wound around a central permeate tube.
- The membrane sheets are separated by spacers, which create flow channels for the feed and permeate streams.
- The feed stream flows through the flow channels in the axial direction, and the permeate stream flows through the flow channels in the radial direction.
- The permeate stream is collected in the central permeate tube and flows out of the module.
- Spiral-wound modules are a very efficient type of membrane module, and they are relatively easy to operate and maintain.
- They are also a relatively low-cost type of membrane module.
Here are some of the advantages of spiral-wound modules:
- High efficiency: Spiral-wound modules are very efficient at separating different components of a mixture.
- Low cost: Spiral-wound modules are relatively low-cost, making them a cost-effective option for many applications.
- Compact size: Spiral-wound modules are compact, making them a good choice for applications where space is limited.
- Easy to operate: Spiral-wound modules are relatively easy to operate, making them a good choice for users with limited experience.
Here are some of the disadvantages of spiral-wound modules:
- Membrane fouling: Spiral-wound modules can be susceptible to membrane fouling, which can reduce their efficiency.
- Membrane cleaning: Spiral-wound modules need to be cleaned regularly to remove foulants.
- Pressure drop: Spiral-wound modules can have a high pressure drop, which can reduce their efficiency.
Overall, spiral-wound modules are a versatile and efficient type of membrane module that is used in a variety of applications. They are a good choice for applications where high efficiency and low cost are important.
Sure, here are some details about tubular modules:
- Tubular modules are a type of membrane module that is used in a variety of membrane processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
- The tubular module consists of a bundle of hollow tubes, each of which is coated with a membrane.
- The feed stream flows through the lumen of the tubes, and the permeate stream flows through the walls of the tubes.
- The permeate stream is collected at the ends of the tubes and flows out of the module.
- Tubular modules are a very efficient type of membrane module, and they are relatively easy to operate and maintain.
- They are also a relatively low-cost type of membrane module.
Here are some of the advantages of tubular modules:
- High efficiency: Tubular modules are very efficient at separating different components of a mixture.
- Low cost: Tubular modules are relatively low-cost, making them a cost-effective option for many applications.
- Long lifespan: Tubular modules have a long lifespan, making them a good choice for applications where long-term operation is required.
- Resilient to fouling: Tubular modules are resilient to fouling, making them a good choice for applications where fouling is a problem.
Here are some of the disadvantages of tubular modules:
- Membrane cleaning: Tubular modules can be difficult to clean, making them a good choice for applications where fouling is not a problem.
- Pressure drop: Tubular modules can have a high pressure drop, which can reduce their efficiency.
- Space requirements: Tubular modules require more space than other types of membrane modules.
Overall, tubular modules are a versatile and efficient type of membrane module that is used in a variety of applications. They are a good choice for applications where high efficiency, low cost, and long lifespan are important.
Here are some additional details about tubular modules:
- The diameter of the tubes in a tubular module typically ranges from 1 to 10 millimeters. The smaller the diameter of the tubes, the higher the efficiency of the module.
- The membrane coating on the tubes in a tubular module is typically made of a polymer or ceramic material. The polymer or ceramic material is selected based on the application and the properties of the feed stream.
- Tubular modules are typically used in applications where the feed stream contains suspended solids or particles. The suspended solids or particles can be trapped on the membrane surface, which can help to protect the membrane from fouling.
Here are some details about hollow-fiber modules:
- Hollow-fiber modules are a type of membrane module that is used in a variety of membrane processes, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis.
- The hollow-fiber module consists of a bundle of hollow fibers, each of which is coated with a membrane.
- The feed stream flows through the lumen of the fibers, and the permeate stream flows through the walls of the fibers.
- The permeate stream is collected at the ends of the fibers and flows out of the module.
- Hollow-fiber modules are a very efficient type of membrane module, and they are relatively easy to operate and maintain.
- They are also a relatively low-cost type of membrane module.
Here are some of the advantages of hollow-fiber modules:
- High efficiency: Hollow-fiber modules are very efficient at separating different components of a mixture.
- Low cost: Hollow-fiber modules are relatively low-cost, making them a cost-effective option for many applications.
- Compact size: Hollow-fiber modules are compact, making them a good choice for applications where space is limited.
- Easy to operate: Hollow-fiber modules are relatively easy to operate, making them a good choice for users with limited experience.
Here are some of the disadvantages of hollow-fiber modules:
- Membrane fouling: Hollow-fiber modules can be susceptible to membrane fouling, which can reduce their efficiency.
- Membrane cleaning: Hollow-fiber modules need to be cleaned regularly to remove foulants.
- Pressure drop: Hollow-fiber modules can have a high pressure drop, which can reduce their efficiency.
Overall, hollow-fiber modules are a versatile and efficient type of membrane module that is used in a variety of applications. They are a good choice for applications where high efficiency and low cost are important.
Here are some additional details about hollow-fiber modules:
- The diameter of the fibers in a hollow-fiber module typically ranges from 0.1 to 1 millimeter. The smaller the diameter of the fibers, the higher the efficiency of the module.
- The membrane coating on the fibers in a hollow-fiber module is typically made of a polymer or ceramic material. The polymer or ceramic material is selected based on the application and the properties of the feed stream.
- Hollow-fiber modules are typically used in applications where the feed stream contains suspended solids or particles. The suspended solids or particles can be trapped on the membrane surface, which can help to protect the membrane from fouling.
Membrane Fouling: Modes of membrane fouling , Control of membrane fouling
Membrane fouling is the accumulation of unwanted materials on the surface or inside the pores of a membrane, which can reduce the membrane's performance and efficiency. There are three main modes of membrane fouling:
- Colloidal fouling is caused by the deposition of colloidal particles on the membrane surface. These particles can be organic or inorganic, and they can be present in the feed water or in the solution being filtered.
- Biofouling is caused by the growth of microorganisms on the membrane surface. This type of fouling is often seen in applications where the feed water contains bacteria, algae, or other microorganisms.
- Chemical fouling is caused by the deposition of dissolved or suspended chemicals on the membrane surface. These chemicals can be organic or inorganic, and they can be present in the feed water or in the solution being filtered.
There are a number of ways to control membrane fouling, including:
- Optimizing the operating conditions. This can include adjusting the flow rate, pressure, temperature, and pH of the feed water.
- Using pre-treatment methods. This can include removing colloidal particles, microorganisms, and chemicals from the feed water before it is filtered.
- Using antifouling membranes. These membranes are designed to resist the deposition of fouling agents.
- Cleaning and regeneration. Membranes that have become fouled can be cleaned or regenerated to restore their performance.
The best way to control membrane fouling depends on the specific application and the type of membrane being used. However, by following these general guidelines, it is possible to minimize fouling and maintain the performance of membrane systems.
Here are some additional tips for controlling membrane fouling:
- Choose the right membrane for the application. Not all membranes are created equal, so it is important to choose a membrane that is designed for the specific application.
- Monitor the feed water quality. The quality of the feed water can have a big impact on the rate of membrane fouling. It is important to monitor the feed water quality and take steps to remove fouling agents before they reach the membrane.
- Backwash the membrane regularly. Backwashing helps to remove foulants from the membrane surface and prevent them from building up.
- Clean the membrane periodically. If the membrane becomes severely fouled, it may need to be cleaned with chemicals.
By following these tips, it is possible to minimize membrane fouling and maintain the performance of membrane systems.
Application of membrane processes:
Membrane processes are used in a wide variety of applications, including:
- Water treatment. Microfiltration, ultrafiltration, nanofiltration, and reverse osmosis are all used to treat water for drinking, industrial, and agricultural purposes. These processes can remove a variety of contaminants from water, including bacteria, viruses, organic matter, and dissolved salts.
- Food and beverage processing. Membrane processes are used to purify and concentrate food and beverage products. For example, microfiltration can be used to remove bacteria from milk, and ultrafiltration can be used to concentrate fruit juices.
- Pharmaceuticals. Membrane processes are used to purify and concentrate pharmaceutical products. For example, nanofiltration can be used to remove impurities from blood plasma, and reverse osmosis can be used to produce ultrapure water for pharmaceutical manufacturing.
- Chemical processing. Membrane processes are used to separate and purify chemicals. For example, microfiltration can be used to remove suspended solids from industrial wastewater, and ultrafiltration can be used to concentrate enzymes.
- Energy production. Membrane processes are used to produce clean energy from water and other sources. For example, reverse osmosis can be used to produce desalinated water for use in power plants, and nanofiltration can be used to produce hydrogen from water.
Here is a table that summarizes the applications of different membrane processes:
| Membrane Process | Pore Size (μm) | Applications |
|---|---|---|
| Microfiltration | 0.1-10 | Water treatment, food and beverage processing, pharmaceutical processing, chemical processing |
| Ultrafiltration | 0.001-0.1 | Water treatment, food and beverage processing, pharmaceutical processing, chemical processing |
| Nanofiltration | 0.0001-0.001 | Water treatment, food and beverage processing, pharmaceutical processing, chemical processing |
| Reverse osmosis | 0.00001-0.0001 | Water treatment, energy production |
As you can see, membrane processes have a wide range of applications. They are used to treat water, purify food and beverages, produce pharmaceuticals, and generate clean energy. Membrane processes are a versatile and efficient way to separate and purify fluids.



.jpg)

0 Comments